Drap orders withdrawal of antibiotic drip Anarob over harmful bacterial toxins
Web Desk
|
9 Sep 2025
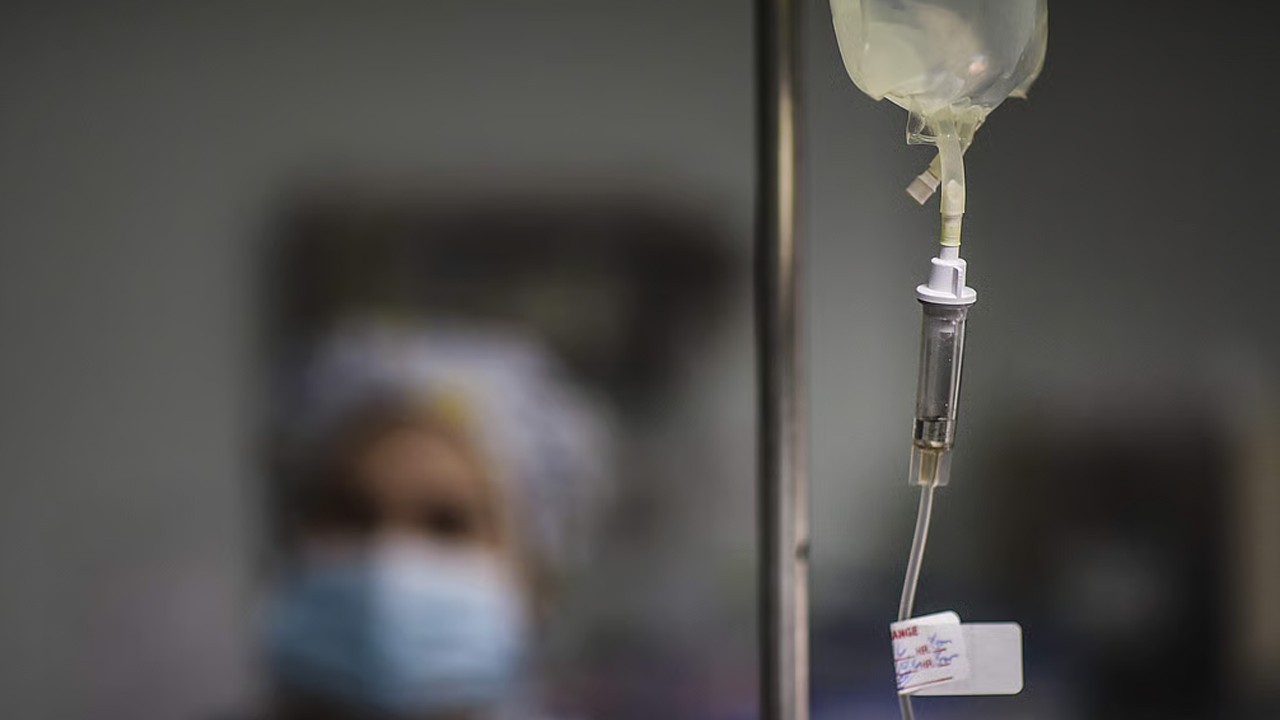
The Drug Regulatory Authority of Pakistan (Drap) on Tuesday ordered the recall of a batch of Anarob antibiotic drip after it was found contaminated with bacterial toxins.
Anarob drip, which contains metronidazole, is administered intravenously to treat severe bacterial infections in the stomach, lungs, skin, joints, and other parts of the body when oral medication is not effective.
According to a medical product alert, Drap’s Central Drugs Laboratory declared samples of the drug “substandard” after detecting excessive levels of bacterial endotoxins.
“Use of this contaminated infusion may cause severe adverse reactions such as fever, chills, septic shock and life-threatening complications. Hospitalised and immunocompromised patients are at the greatest risk,” the authority cautioned.
The regulator directed its field teams and provincial drug control departments to conduct immediate market surveys and remove the affected batch (H24219) from circulation. It also instructed authorities across all federating units to step up surveillance to ensure the recall is effectively enforced.
Also Read: DRAP recalls toxic cough syrup after pharmaceutical company in Peshwar reported to DRAP
Pharmacists and chemists working at distribution centres and pharmacies have been told to check their stocks, stop supplying the product, and quarantine remaining vials before returning them to the supplier. Drap also called for “increased vigilance within the supply chains of institutions, pharmacies and healthcare facilities” that may have received the batch.
The authority urged that any adverse reactions or quality issues linked to the drug be reported to the National Pharmacovigilance Centre (NPC) through the Adverse Event Reporting Form or its online portal.
“Consumers should stop using products bearing the affected batch number and shall contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product, and report the incident to Drap/National Pharmacovigilance Centre,” the alert concluded.












Comments
0 comment